Selective Catalytic Reduction (SCR) is a process where nitrogen oxides (NOx) are converted to diatomic nitrogen (Nx) and water (H2O) through a reaction that is caused by adding a reductant such as ammonia (NH3 or NH4OH) or urea [CO(NH2)2] to the steam of flue.
As SCRs are forced to operate at lower temperatures, ammonia bisulfate is more likely to condense on catalyst surfaces causing fouling, pluggage, and issues with reaching target DeNox rates. Over-injection of ammonia becomes more and more necessary. Ammonia slip measurements have traditionally been used as they relate to SCR performance for NOx reduction and to minimize over injection for economic reasons as well as to minimize air heater plugging, but ammonia concentration is also strongly related to mercury oxidation. In the presence of NH3, competitive adsorption between NH3 and elemental Hg on SCR catalyst is partly responsible for weakening Hg oxidation. NH3 consumes surface oxygen (O2) on the catalyst, which also results in lower Hg oxidation rates, especially in the absence of gas phase O2 in the flue gas. More importantly, oxidized mercury can be reduced by NH3 to form elemental Hg with the aid of NO, i.e., the combined presence of NO and NH3 induces the reduction of oxidized mercury, and hence partially offsets Hg oxidation. (source)
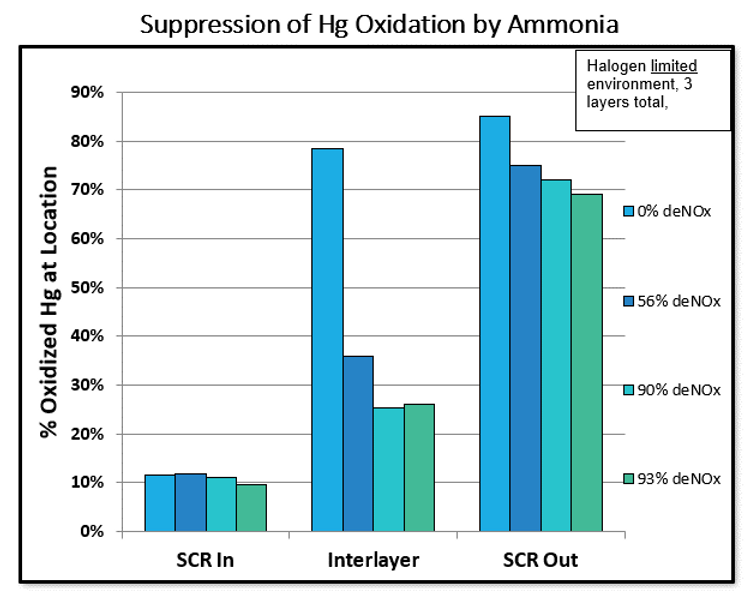
This graph’s four colors represent different DeNOx percentages, including 0, 56, 90, and 93. As expected, higher DeNOx results in lower mercury oxidation. This is because Ammonia donates electrons to another species with a higher oxidation state (the oxidizing agent) and reduces it. The nitrogen atom in ammonia has an oxidation state of -3, which means NH3 can act as a reducing agent, losing electrons to another species with a higher oxidation state. Thereby, the presence of excess ammonia can decrease the effectiveness of certain mercury control technologies such as dry sorbent injection (DSI), and wet FGD, which are typically used to control primarily oxidized Hg(2+) emissions. Additionally, we can see that most mercury oxidation occurs on the last layer of the catalyst if you look at the difference between the interlayer and SCR outlet mercury oxidation percentages. The point again is to illustrate how ammonia and mercury are linked, and how direct ammonia measurements are a useful tool in diagnosing problems with the catalyst and optimizing its performance.
If your flue gas measurements are showing a higher-than-expected level of Hg at the stack, evaluating your ammonia slip is important since ammonia can dramatically affect mercury oxidation potential. The best way to analyze this is not with models but by incorporating actual measurements into your evaluation. Traditionally, this data has been tricky to obtain because the available measurement methods and equipment are cumbersome and expensive. However, our goal is to make your life easier by providing user-friendly measurement tools at a fraction of the cost.
Ammonia Sorbent Traps can be used to easily measure your Ammonia Slip at various operating parameters. Use them to determine SCR deficiencies, prevent over-injection, and track deactivation over time which can help you to predict catalyst failure. We have even used ammonia sorbent traps to perform interlayer testing to determine rate of degradation for each layer.
Below is an example of some test data we collected for one of our customers while doing an SCR test with four pairs of traps, one for each catalyst layer.
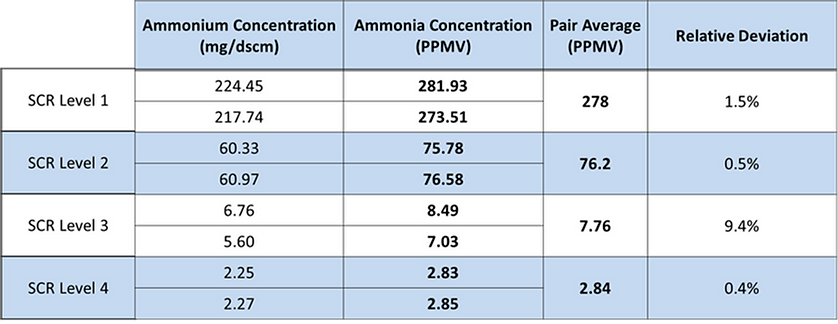
- You can see a pretty dramatic drop in ammonia as the gas passes through each layer of the SCR, until finally we end up with about 2.8 ppm of ammonia leaving the SCR.
- This is the sort of SCR profile that can be incorporated into a modeling and management plan to get a better sense of what is going on with each individual layer.
- The slip in this case was within the expected range of 2 to 3 ppm, but one thing we’ve noticed in the testing that we have done so far is that the ammonia slip is rarely what the plant expects. The theoretical value provided by models or catalyst manufacturers is often off by a significant amount.
- This is why incorporating actual field measurements into the models can greatly improve the reliability of those models and improve the SCR management program overall.
The traps are capable of measuring very low concentrations of ammonia with a high degree of accuracy. Normal sample runs are approximately 20 minutes long, but with an extended duration such as two hours, it is possible to reach very low concentrations. Our current MDL is 0.06ppmvd NH3 in flue gas, with a quantification limit of 0.25ppmvd with extended sampling. The sorbent trap method is truly superior to other ammonia measurement methods. To read more about this, see our blog post on Advantages of Measuring Ammonia using Sorbent Traps.
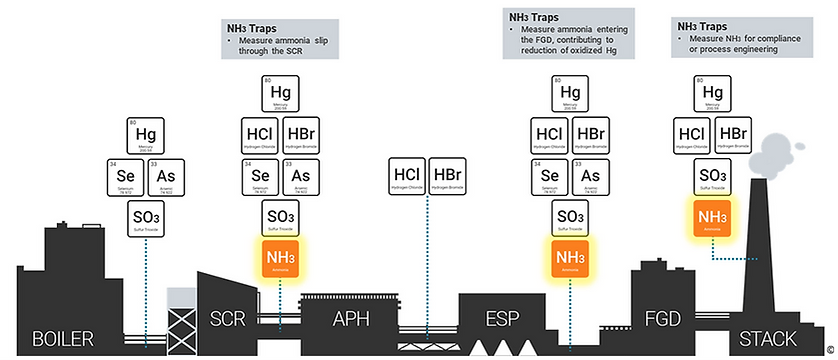
Simultaneously sampling Mercury Speciation Sorbent Traps is also helpful in this evaluation. Typically, it is the 4th or last layer of catalyst where the majority of mercury oxidation reactions take place. Understanding the efficiency of the SCR is very important in uncovering ways to optimize its performance and further identify what is impacting your mercury control. Just be sure to use a cooling probe with a fine temperature control kit and have the speciation traps analyzed on-site as you will need to optimize your sampling parameters to account for the high temperatures around the SCR as well as the high particulate loading. Oh, and we rent these sampling systems and analyzers in case you were curious…
Next Up…arsenic and how it affects mercury control!
Miss part of the series? Check out a few of our other blog posts on elevated Hg levels:

